Theme
Generating liver and pancreatic tissues from human iPS cells for regenerative medicine through understanding of the mechanisms of organ development.
About Research
Developing liver and pancreatic tissues from iPSC
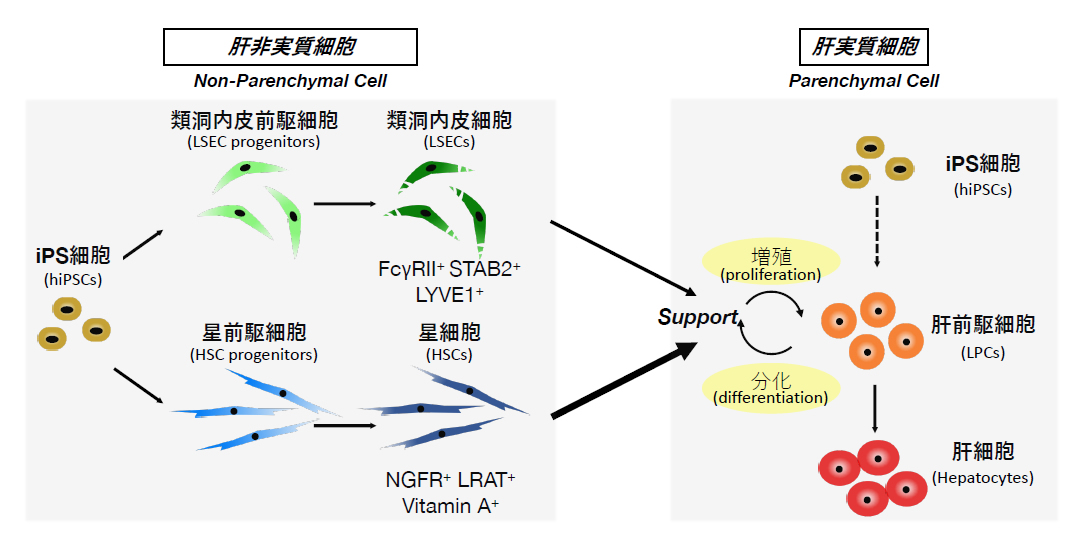
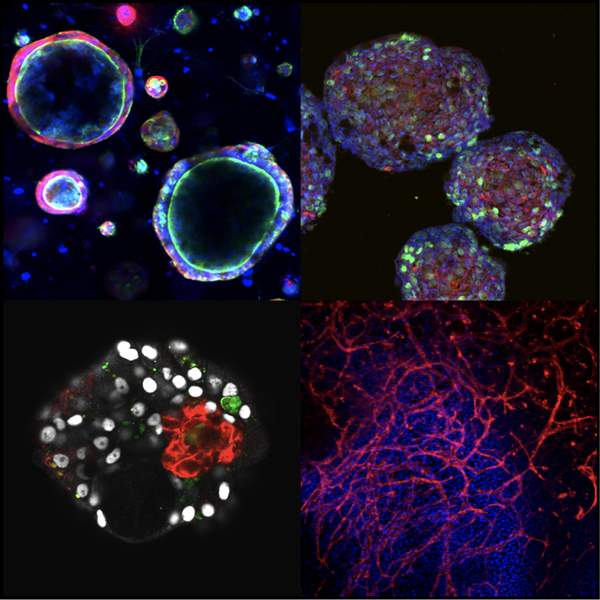
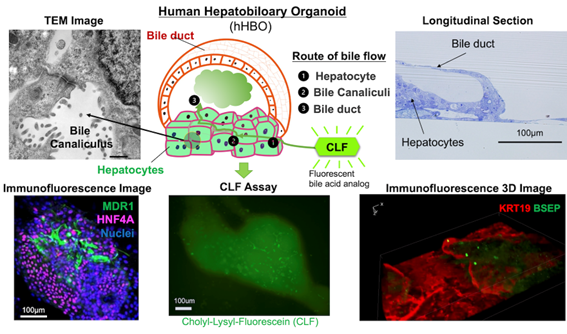
Development of systems to prepare functional cells and tissues of sufficient quality and quantity is central to realization of stem cell-based therapy, drug screening and regenerative medicine. The liver is a central organ for metabolism and detoxification in the body and hence is one of the major targets for those applications. For the last two decades, our lab has been studying the molecular and cellular mechanisms underlying development of the liver, taking advantage of our originally developed methods to isolate and culture each type of liver omponent cells. During the course of liver organogenesis, parenchymal hepatocytes develop from fetal liver progenitor cells, also known as hepatoblasts, through multiple modes of cell–cell interactions with other types of non-arenchymal cell populations including endothelial cells and mesenchymal cells. Based on our accumulated knowledge and expertise on the mechanistic basis of the liver development, we have successfully established culture systems to achieve directed differentiation of functional hepatocytes, as well as other non-parenchymal cells, from human induced pluripotent stem cells (iPSCs) through corresponding progenitor cell populations. Further application of three-dimensional co-culture system have rendered those iPSC-derived liver cells to cooperatively organize functional liver tissues with remarkable metabolic activities. Similarly, we have also developed a culture system to generate pancreatic islet-like tissue structures containing insulin-producing β cells from human iPSCs. We are currently applying those iPSC-derived cells and tissues for regenerative medicine, drug discovery and disease
modeling.
Publication
- Nakano Y, Saijou E, Itoh T, Tanaka M, Miyajima A, Kido T. Development of a high throughput system to screen compounds that revert the activated hepatic stellate cells to a quiescent-like state. Sci Rep. 2024. doi: 10.1038/s41598-024-58989-6.
- Koui Y, Kido T. Using human induced pluripotent stem cell-derived liver cells to investigate the mechanisms of liver fibrosis in vitro. Biochem Soc Trans. 28;51(3):1271-1277. 2023. doi: 10.1042/BST20221421.
- Himeno M, Chen SW, Kido T. Co-culture model for hepatitis B virus infection using. iPSC-derived liver progenitor cells and liver sinusoidal endothelial cells. Methods Mol Biol 2544:107-117. 2022. doi: 10.1007/978-1-0716-2557-6_7.
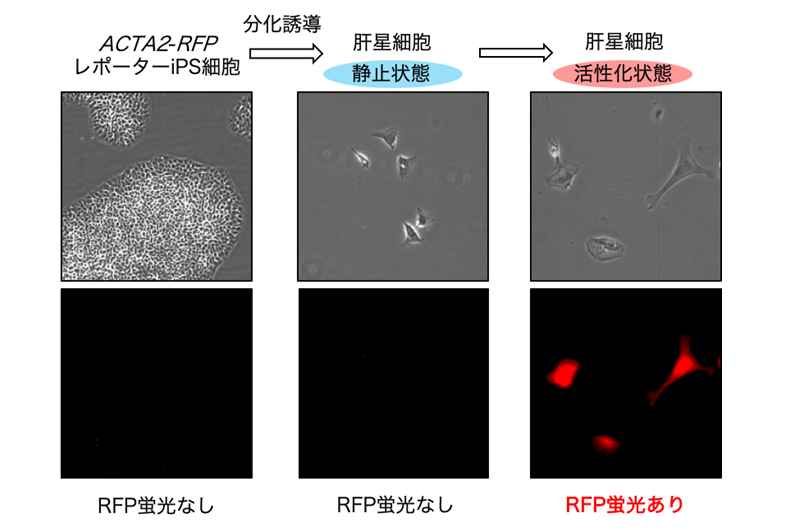
- Koui Y, Himeno M, Mori Y, Nakano Y, Saijou E, Tanimizu N, Kamiya Y, Anzai H, Maeda N, Wang L, Yamada T, Sakai Y, Nakato R, Miyajima A, and Kido T. Development of human iPSC-derived quiescent hepatic stellate cell-like cells for drug discovery and in vitro disease modeling. Stem Cell Rep. 16, 3050–3063, 2021. https://doi.org/10.1016/j.stemcr.2021.11.002
- Watanabe A, Tanaka A, Koga C, Matsumoto M, Okazaki Y, Kin T, and Miyajima A. CD82 is a marker to isolate β cell precursors from human iPS cells and plays a role for the maturation of β cells. Sci Rep. 2021 May, 11: 9350. https://doi.org/10.1038/s41598-021-88978-y
- Naoki Tanimizu, Norihisa Ichinohe, Yasushi Sasaki, Tohru Itoh, Ryo Sudo, Tomoko Yamaguchi, Takeshi Katsuda, Takafumi Ninomiya, Takashi Tokino, Takahiro Ochiya, Atsushi Miyajima, and Toshihiro Mitaka. Generation of functional liver organoids on combining hepatocytes and cholangiocytes with hepatobiliary connections ex vivo. Nature Comm. 2021 Jun 7;12(1):3390. doi: 10.1038/s41467-021-23575-1
- Kamimoto K, Nakano Y, Miyajima A, and Itoh T. Multidimensional imaging of liver injury repair in mice reveals fundamental role of the ductular reaction. Communications Biology (2020) 3: 289. https://doi.org/10.1038/s42003-020-1006-1
- Itoh T and Miyajima A. Filling a gYap in Hepato-Biliary Tissue Integration in Liver Homeostasis and Regeneration. Cell Stem Cell. 2019 Jul 3;25(1):5-6. doi: 10.1016/j.stem.2019.06.006.
- Miura Y, Matsui S, Miyata N, Harada K, Kikkawa Y, Ohmuraya M, Araki K, Tsurusaki S, Okochi H, Goda N, Miyajima A. and Tanaka M. Differential expression of Lutheran/BCAM regulates biliary tissue remodeling in ductular reaction during liver regeneration. eLife 2018; 7: e 36572. Doi.org/10.7554/eLife.36572
- Saijou E, Enomoto Y, Matsuda M, Kok C, Akira S, Tanaka M and Miyajima A. Neutrophils alleviate fibrosis in the CCl4-induced mouse chronic liver injury model. Hepatology Communications.2, 703-717, 2018. DOI 10.1002/hep4.1178
Atsushi Miyajima
Project Professor
Ph.D.
Taketomo Kido
Project Associate Professor
Ph.D.