Theme
Our laboratory focuses on the role of epigenetics (the memory system of cells) in the brain (the memory system of individuals). In the future, our research will contribute to understanding the mechanisms of functional changes in the brain caused by aging, stress, and psychiatric disorders.
About Research
Understanding how the brain functions through epigenetic analysis
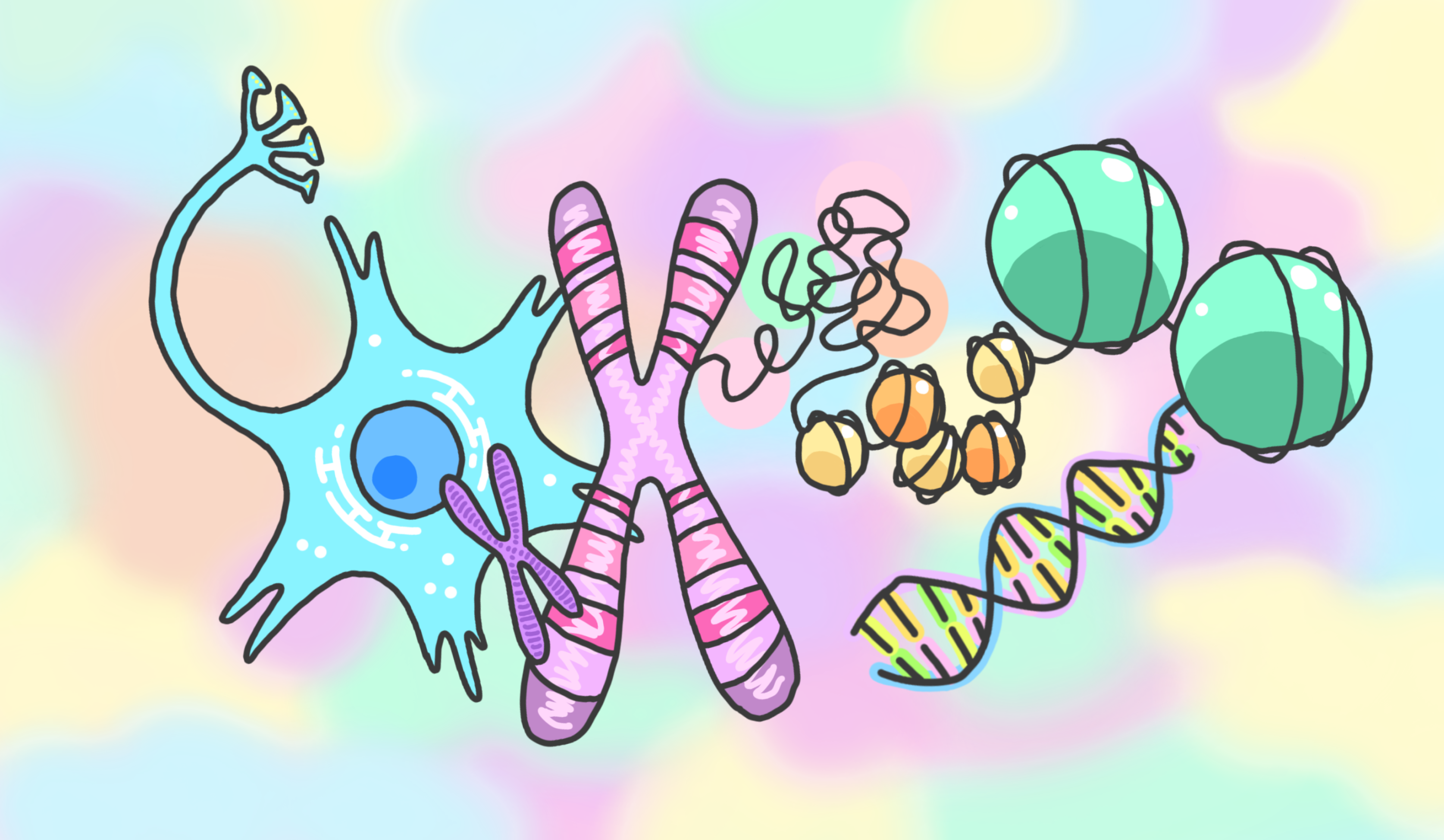
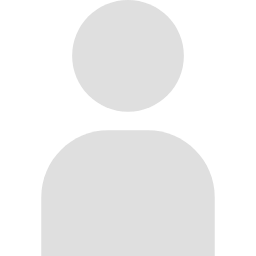
Our genetic information is encoded in our genomic DNA. Several mechanisms are used to properly identify and access the necessary genetic information contained within our three billion base pairs of DNA. One of these mechanisms is epigenetic regulation. Through epigenetic regulation, chemical modifications on DNA and histones—such as methylation and acetylation—function as “bookmarks,” resulting in the expression of only the necessary genes.
These epigenetic “bookmarks” are modified in response to the cell’s previous exposures to stimuli and experiences. In other words, epigenetic regulation functions as the memory system of cells. The question then becomes, what role does epigenetics play in the brain, which is the memory system of individuals?
In our laboratory, we are working to understand the mysteries of the brain through epigenetic analysis by combining the biochemical, molecular biological, and bioinformatics technologies needed to carry out the latest genomic analysis with the genetic and neuroscience technologies necessary to analyze brain function. Through this research, we aim to understand the basis of changes in brain function caused by aging, stress, and psychiatric disorders.
Publication
- Kishi Y*, Gotoh Y. (2021) Isolation of genetically manipulated neural progenitors and immature neurons from embryonic mouse neocortex by FACS. STAR protocol 2:100540
- Eto H and Kishi Y*. (2021) Brain regionalization by Polycomb-group proteins and chromatin accessibility. BioEssays 43(11):e2100155
- Eto H†, Kishi Y†*, Yakushiji-Kaminatsui N, Sugishita H, Utsunomiya S, Koseki H, Gotoh Y*. (2020) The Polycomb group protein Ring1 regulates dorsoventral patterning of the mouse telencephalon. Nature Communications 11:5709
- Nagahama K, Sakoori K, Watanabe T, Kishi Y, Kawaji K, Koebis M, Nakao K, Gotoh Y, Aiba A, Uesaka N*, Kano M*. (2020) Setd1a Insufficiency in Mice Attenuates Excitatory Synaptic Function and Recapitulates Schizophrenia-related behavioral abnormalities. Cell Reports 15;32(11):108126
- Nakagawa T, Wada Y, Katada S*, Kishi Y*. (2020) Epigenetic regulation for acquiring glial identity by neural stem cells during cortical development. GLIA 68(8):1554
- Sakai H†, Fujii Y†, Kuwayama N, Kawaji K, Gotoh Y*, Kishi Y†*. (2019) Plag1 regulates neuronal gene expression and neuronal differentiation of neocortical neural progenitor cells. Genes to Cells 24(10):650-666
- Tsuboi M†, Kishi Y†*, Kyozuka W, Koseki H, Hirabayashi Y, Gotoh Y*. (2018) Ubiquitination-independent repression of PRC1 targets during neuronal fate restriction in the developing mouse neocortex. Developmental cell 47:758–772
- Kishi Y*, Gotoh Y. (2018) Regulation of Chromatin Structure During Neural Development. Frontiers in Neuroscience 12:874
- Fujii Y, Kishi Y, Gotoh Y*. (2013) IMP2 regulates differentiation potentials of mouse neocortical neural precursor cells. Genes to Cells 18(2):79-89
- Kishi Y, Fujii Y, Hirabayashi Y, Gotoh Y*. (2012) HMGA regulates the global chromatin state and neurogenic potential in neocortical precursor cells. Nature Neuroscience 15:1127-1133
Yusuke Kishi
Associate Professor
Ph.D.
Graduate School of Pharmaceutical Sciences
Merve Bilgic
Reseach Associate
Ph.D.