Theme
The acquired immune system centered around T cells is essential for maintaining the homeostasis of the individual. However, excessive activation of the immune system leads to inflammatory diseases and autoimmune disorders. We aim to understand the fundamental mechanisms of lymphocyte activation and tolerance and develop novel therapeutic approaches.
About Research
Our lab aims to understand underlysing mechanisms of inflammation and autoimmune diseases
The acquired immune system, centered around T cells, is essential for maintaining the homeostasis of an individual through the elimination of pathogens. However, excessive activation of the immune system can lead to inflammatory and autoimmune diseases. T cells in the body interact with self-cells and constantly receive weak survival signals from antigen receptors, thus multiple mechanisms exist to avoid unnecessary activation.
We have pioneered in elucidating the function of co-stimulatory molecules (CD28, CTLA-4, PD-1) fundamentally involved in T cell tolerance. Additionally, we have discovered that the TRIM28 molecule, involved in gene expression control in the cell nucleus, is closely associated with peripheral immune tolerance, metabolic disorders, and individual aging through chromatin regulation, energy metabolism, activation, and aging of immune cells.
In this field, we are advancing research by biochemically and immunologically analyzing these immune tolerance-related molecules, aiming to understand fundamental activation and inhibition mechanisms of T cells and to develop novel therapeutic approaches based on this knowledge.
Publication
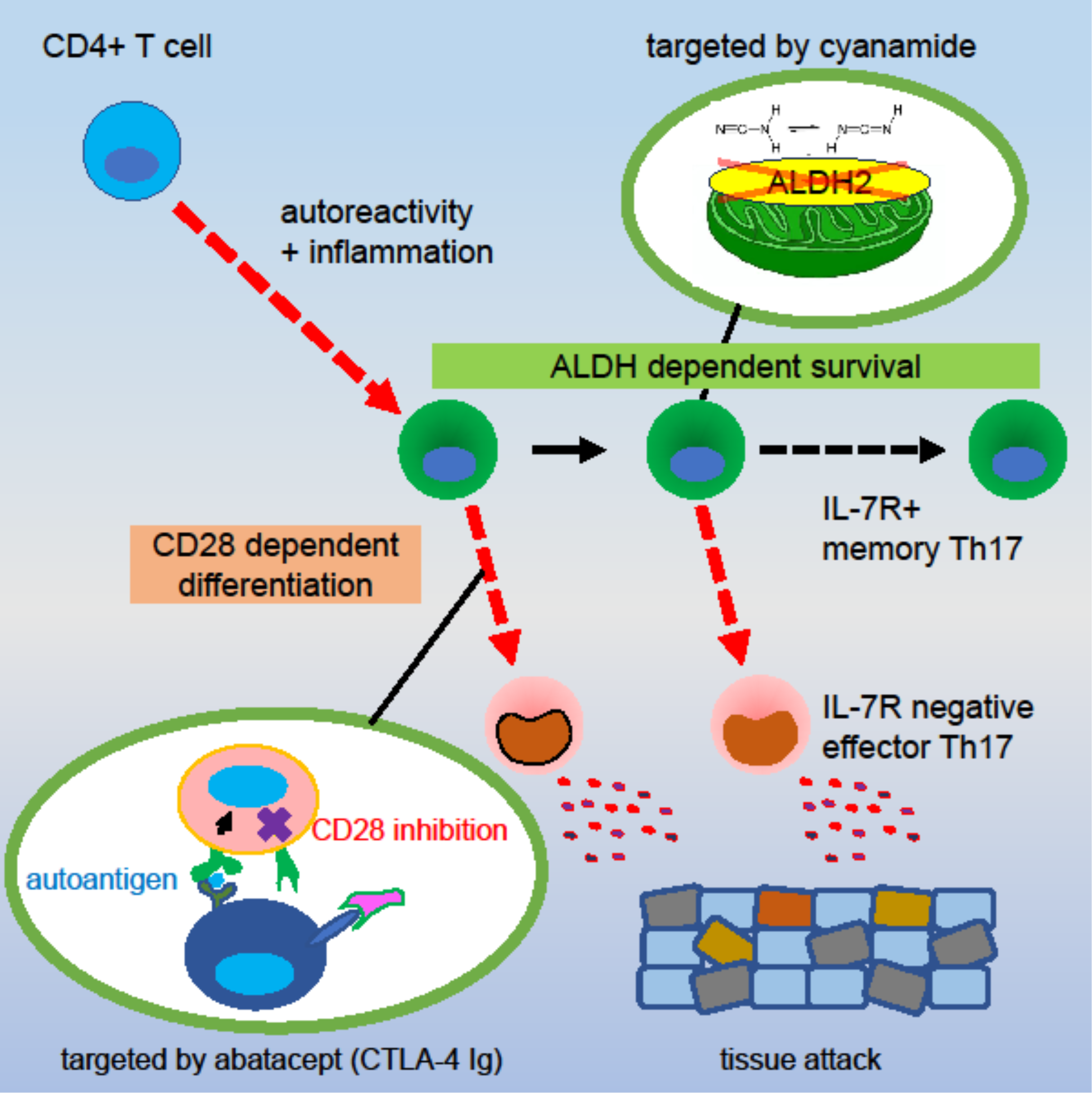
- Tokifuji Y, Hayabuchi H, Sasaki T, Hara-Chikuma M, Hirota K, Takahashi H, Amagai M, Yoshimura A, Chikuma S (Corresponding Author.) Targeting abatacept-resistant T-helper-17 cells by aldehyde dehydrogenase inhibition. iScience, 27: 108646, 2024.
- Miyamoto K, Hayabuchi H, Tokifuji Y, Ando M, Onishi N, Okamura T, Yoshimura A, Chikuma S. (Corresponding Author) A Protein Kinase D inhibitor suppresses AKT on T cells and antagonizes cancer immunotherapy by Anti-PD-1. Int Immunol, 16, dxac035, 2022.
- Chikuma S (Corresponding Author), Yamanaka S, Nakagawa S, Ueda MT, Hayabuchi H, Tokifuji Y, Kanayama M, Okamura T, Arase H, Yoshimura A. TRIM28 expression on dendritic cells prevents excessive T cell priming by silencing endogenous retrovirus. J Immunol, 206:1528-1539, 2021.
- Chikuma S. (Corresponding Author) CTLA-4, an Essential Immune-Checkpoint for T cell Activation. Curr Top Microbiol Immunol, 410: 99-126, 2017.
- Okada M, Chikuma S, Kondo T, Hibino S, Machiyama H, Yokosuka T, Nakano M, Yoshimura A.
Blockage of Core Fucosylation Reduces Cell-Surface Expression of PD-1 and Promotes Anti-tumor Immune Responses of T Cells. Cell Rep, 20(5):1017-1028, 2017.
- Zhang B †, Chikuma S †, Hori S, Fagarasan S, Honjo T*. Nonoverlapping roles of PD-1 and FoxP3 in maintaining immune tolerance in a novel autoimmune pancreatitis mouse model. Proc Natl Acad Sci U S A, 113(30):8490-5, 2016 (†: equal contribution)
- Rui Y, Honjo T, Chikuma S. Programmed cell death 1 inhibits inflammatory helper T-cell development through controlling the innate immune response. Proc Natl Acad Sci U S A, 110(40):16073-8, 2013.
- Chikuma S, Suita N, Okazaki IM, Shibayama S, Honjo T. TRIM28 prevents autoinflammatory T cell development in vivo.
Nat Immunol, 13:596-603, 2012.
- Terawaki S †, Chikuma S †, Shibayama S †, Hayashi T, Yoshida T, Okazaki T, Honjo T. IFN-α directly promotes programmed cell death-1 transcription and limits the duration of T cell-mediated immunity. J. Immunol, 186: 2772-9. 2011. (†: equal contribution)
- Chikuma S. (Corresponding Author) Basics of PD-1 in self-tolerance, infection, and cancer immunity. Int J Clin Oncol. 21: 448-455, 2016.
Shunsuke Chikuma
Visiting Associate Professor
Ph. D.