Most genetic information encoded by the genomic DNA is first transcribed as messenger RNAs (mRNAs), followed by translation to proteins to exert their functions. Coined by Francis Crick in 1958, this flow of genetic information―called the Central Dogma―has been widely accepted as a basic principle in molecular biology. However, recent studies have revealed many important exceptions to this principle. Our laboratory is investigating one such exception called non-coding RNAs (ncRNAs), which act as functional RNA molecules without being translated to proteins.
Well-known ncRNAs such as rRNAs (ribosomal RNAs), tRNAs (transfer RNAs) and snRNAs (small nuclear RNA) were all discovered at the dawn of molecular biology. These canonical ncRNAs play pivotal roles in fundamental processes of the Central Dogma including mRNA processing and translation, and as such, their functions and actions have been studied extensively. However, recent studies revealed that a much wider variety of ncRNA species are in fact expressed in eukaryotic cells. For instance, miRNAs (microRNAs), siRNAs (small interfering RNAs) and piRNAs (piwi-interacting RNAs) are tiny ncRNAs of 20-30 nucleotides discovered from the 1990's onward. These small RNAs recognize their target mRNAs through base pairing and regulate the fundamental flow of the Central Dogma at post-transcriptional and transcriptional levels. More recently, transcriptome analyses have identified numerous long non-coding RNAs (lncRNAs) with diverse functions including epigenetic regulation. These newly discovered ncRNAs are thought to play essential roles in complex biological processes by dynamically and finely modulating gene expression. Yet, our knowledge on production and function of these ncRNA species is still very limited. We are challenging this new frontier of the RNA world by combining biochemistry, biophysics, and cellular and developmental biology.
Assembly of ncRNA machineries
Most ncRNAs undergo post-transcriptional processing and exert their functions by forming ribonucleoprotein complex with their partner proteins. For example, small RNAs are excised from their precursor RNAs and loaded into Argonaute family proteins (Ago) in a well-ordered manner. This is achieved by coordinated actions of multiple protein factors. We aim to dissect the mechanisms of the assembly of ncRNA machineries by combining in vitro reconstitution system and single-molecule imaging.
Actions of ncRNA machineries
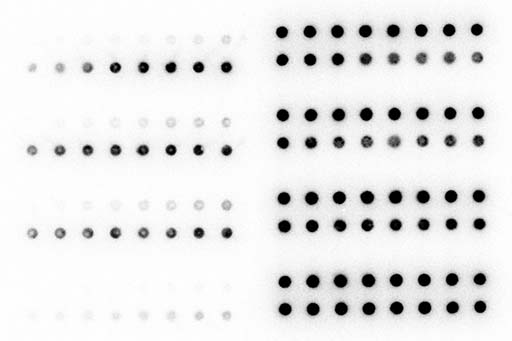
Many ncRNAs act as guide molecules for ncRNA machineries to select their target RNAs. In animals, for example, each miRNA binds hundreds of different target mRNAs by recognizing a sequence of six to eight nucleotides in length. miRNAs inhibit translation and promote degradation of those target mRNAs, thereby controlling a wide range of gene expression. However, it is still poorly understood exactly how ncRNA machineries regulate their target RNAs. We aim to dissect molecular actions of ncRNA machineries biochemically, and understand their roles in the context of living cells and organisms.