Theme
Our aim is to elucidate the regulatory mechanisms underlying tissue remodeling (e.g. ‘regeneration’ and ‘fibrosis’) in liver diseases from the perspective of cell-cell interaction for the development of novel diagnostic and therapeutic methods.
About Research
Elucidation of intercellular interactions relevant to liver regeneration and fi brosis in liver diseases.
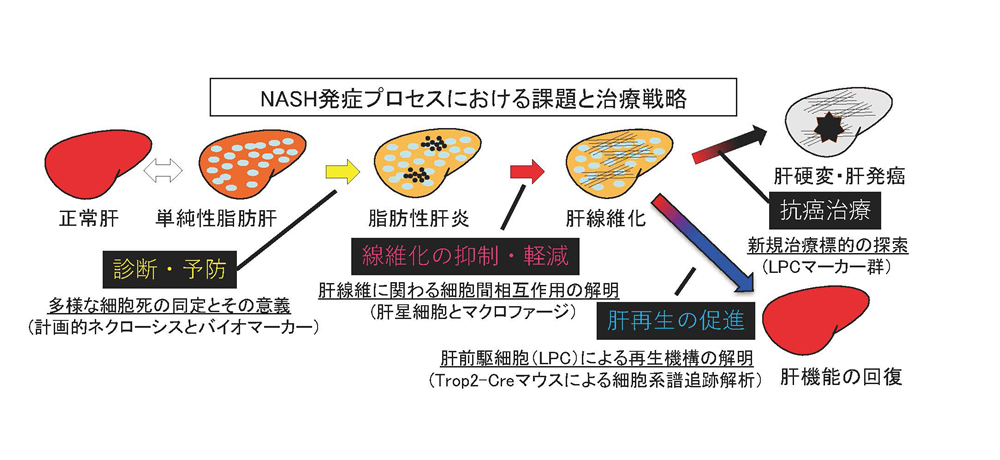
Liver is known to regenerate upon drug-induced acute injury or hepatectomy. However, when liver suff ered from prolonged viral infection or alcohol intake, impaired regeneration could cause fi brosis with excess collagen accumulation in the liver, eventually leading to cirrhosis and carcinogenesis. Liver is composed of hepatocytes and non-parenchymal cells including sinusoidal endothelial cells, hepatic stellate cells, biliary epithelial cells, blood cells and so on. Although these cells communicate adequately with each other at steady state, forming “cell society”, disordered communication could cause severe disease state. Therefore, we focus on the pathology of non-alcoholic steatohepatitis (NASH), and aim to elucidate the cell-cell interaction involved in the regulation of each progression step from hepatic cell death to carcinogenesis. For the purpose of developing novel therapy and regenerative medicine in chronic liver diseases, we especially promote research on two liver remodeling, i.e. ‘liver fibrosis’ and ‘liver stem/progenitor cell-mediated regeneration’.
Publication
- Differential expression of Lutheran/BCAM regulates biliary tissue remodeling in ductular reaction during liver regeneration. Miura Y, Matsui S, Miyata N, Harada K. Kikkawa Y, Ohmuraya M, Araki K, Tsurusaki S, Okochi H, Goda N, Miyajima A, *Tanaka M. Elife. 7. pii: e36572. 2018
- Characterization of Peribiliary Gland-Constituting Cells Based on Differential Expression of Trophoblast Cell Surface Protein 2 in Biliary Tract. Matsui S, Harada K, Miyata N, Okochi H, Miyajima A, *Tanaka M. Am J Pathol. 188(9):2059-2073. 2018
- Oncostatin M causes liver fibrosis by regulating cooperation between hepatic stellate cells and macrophages in mice. Matsuda M, Tsurusaki S, Miyata N, Saijou E, Okochi H, Miyajima A, *Tanaka M. Hepatology. 67(1):296-312. 2018
- Oncostatin M maintains the hematopoietic microenvironment in the bone marrow by modulating adipogenesis and osteogenesis. Sato F, Miyaoka Y, Miyajima A, *Tanaka M. PLoS One 9:e116209. 2014
- Semaphorin 3E secreted by damaged hepatocytes regulates the sinusoidal regeneration and liver fibrosis during liver regeneration. Yagai T, Miyajima A, *Tanaka M. Am J Pathol. 184: 2250-9. 2014
- Stem/progenitor cells in liver development, homeostasis, regeneration, and reprogramming. *Miyajima A, *Tanaka M, *Itoh T. Cell Stem Cell 14:561-74. 2014
- Characterization and functional analyses of hepatic mesothelial cells in mouse liver development. Onitsuka I, Tanaka M, *Miyajima A. Gastroenterology 138:1525-35. 2010
- Potential hepatic stem cells reside in EpCAM+ cells of normal and injured mouse livers. Okabe M, Tsukahara Y, *Tanaka M, Suzuki K, Saito S, Kamiya Y, Tsujimura T, Nakamura K, Miyajima A. Development 136:1951-60. 2009
- p75 Neurotrophin receptor is a marker for precursors of stellate cells and portal fibroblasts in mouse fetal liver. Suzuki K, *Tanaka M, Watanabe N, Saito S, Nonaka H, Miyajima A. Gastroenterology 135:270-281. 2008
- Targeted disruption of Oncostatin M receptor results in altered hematopoiesis. *Tanaka M, Hirabayashi Y, Sekiguchi T, Inoue T, Katsuki M, Miyajima A. Blood 102:3154-62. 2003
Minoru Tanaka
Associate Professor
博士(農学)
新領域創成科学研究科・メディカル情報生命専攻